ENERGY BANDS
A matter is composed of small particles known as atoms. When the atoms combine to form a molecule, the electron shell is influenced not only by its now nucleus but also by the nuclei and electrons of surrounding atoms. It has been observed that nearest atoms have the greatest influences over the energy level (electron shell) of an atom. Thus the energy level of each electron in a given atom is controlled, to some extent, by all the neighbouring atoms is the molecule.
It will be interesting to know that the energy levels of all electrons, in a molecule, are different. But the electrons readjust themselves in such a way that their energy levels come closer to each other. Or in other words all electrons, in the molecule have their first energy levels in the vicinity of each other, which form a cluster or band known as energy band. Similarly, all the electrons in the second shell form the second energy band, corresponding to the outermost shell, is called the valence band as shown in fig. 8.
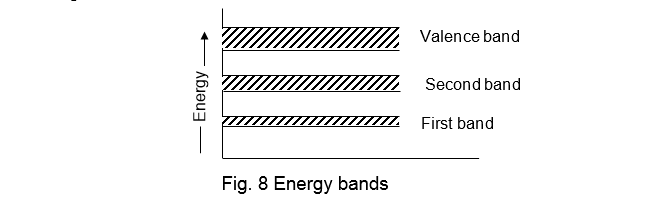
It has been observed that when the valence electrons are removed from their respective atoms, they are called free or condition electrons. The total energy of a free electron is slightly higher than that in the valence orbit. It is due to this fact that a valence electron absorbs energy at the time of leaving its atom. Therefore the energy of a free electron is very close to the valence level.
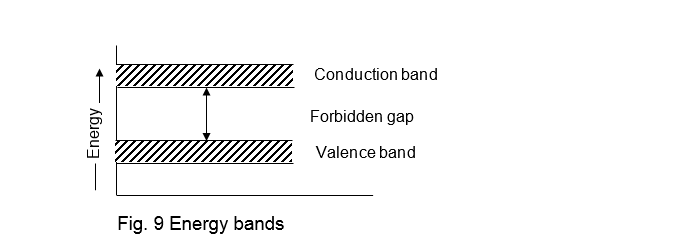
The energy formed a molecule by the conduction levels of various atoms is known as conduction band. It is represented in the energy band diagram above the valence band. The energy gap between the conduction band and the valence band is known as forbidden gap as shown in fig. 9. It may be noted that there is no energy shell in this forbidden gap, which the electron can occupy.
Classification of Solids Based on Band Theory
The solids may also be classified on the basis of band theory into the following three categories
- Conductors
- Insulators
- Semiconductors
- Conductors
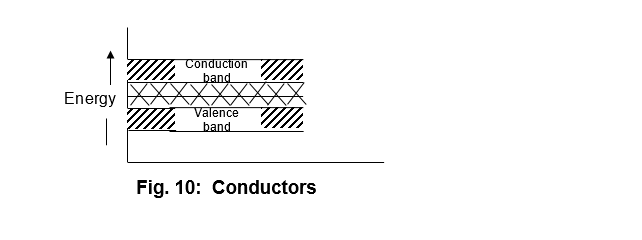
These are the solids in which the valence band overlaps the conduction band as shown in fig. 10
It may be noted that due to overlapping of the two bands, the electrons require a slight amount of energy to jump from the valence band to the conduction band, and thus the solids are known as conductors. The examples of such solids are silver, aluminium etc.
- Insulators
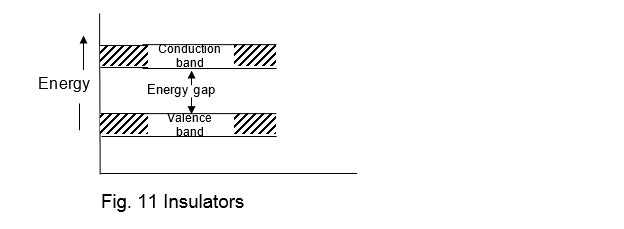
These are the solids in which there exists a large energy gap (i.e. forbidden gap) in between the valence band and conduction band as shown in fig 11.
It may be noted that due to the large energy gap between the two bands, the electrons cannot jump from the valence band to the conduction band (which is empty) at ordinary temperatures. However, they require a large amount of energy to jump. Therefore the electrical conduction in insulators cannot take place. It has been observed that if the temperature of an insulator is increased, some electrons do jump to the conduction band. Therefore a small electrical conduction may take place. The examples of such solids are rubber, Bakelite, mica etc.
- Semiconductors
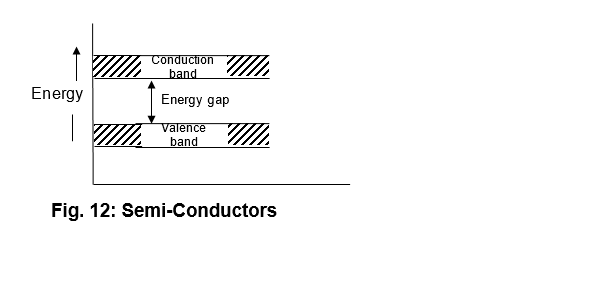
These are solids in which there exists a small energy gap (i.e. forbidden gap) in between the valence band and conduction band as shown in fig. 12.
It may be noted that due to the small energy gap between the two bands, no electron can jump from the valence band to the conduction band (which is empty) at absolute zero temperature (i.e. OK). However, as the temperature is increased say up to room temperature, some of the electrons jump into the conduction band. Thus in a semiconductor the conductivity (or flow of current) increases with the increase in temperature. Moreover, the electrical conductivity in such materials lies in between those of conductors and insulators. The examples of solids in this category are germanium and silicon.
Superconductivity
The term “superconductivity” may be defined as a state of material in which it has zero resistivity. The resistivity of all materials increases with the temperature. Or in other words, if the material is cooled below the room temperature, the resistivity decreases with the decrease in temperature. Prof. Heike discovered in 1911 that mercury is a good conductor of electricity at room temperature. Its resistivity decreases slightly with the decrease in temperature up to 4.27k. Beyond this temperature, its resistivity decreases sharply and finally zero at 4.22k. This transition occurs a very narrow range of temperature of the other of 0.05kas shown in fig. 13
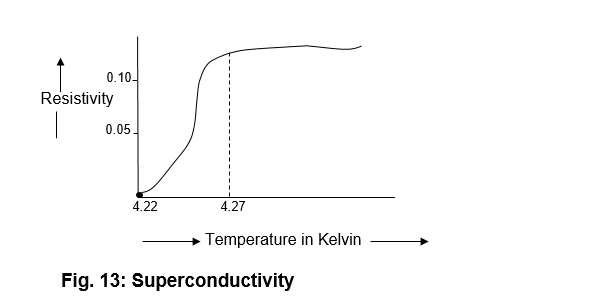
As a matter of fact, the resistivity of mercury is a function of temperature. The temperature at which there is a sudden transition temperature.
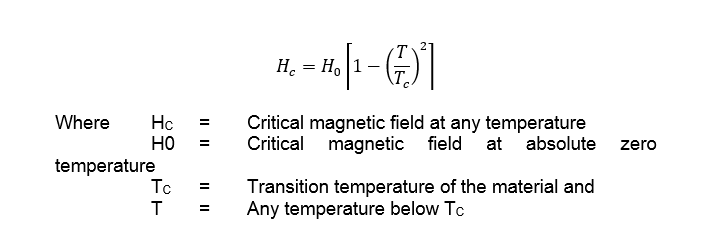
It has been observed that only a few metals such as tin, Lead, tantalum, bismuth, antimony, tellurium etc show superconductivity. It will be interesting to know, that all the above mentioned metals are poor conductors of electricity at room temperature. On the other hand, the metals such as copper, silver and gold which are good conductor of electricity at room temperature do not show superconductivity. The transition temperature of superconducting metal may range from 0.01k to 9.15k. It has also been observed that the superconductivity of the materials may be destroyed by the application of sufficiently strong magnetic field. The critical value of magnetic field, for the destruction of superconductivity, is a function of temperature. It varies according to the following relation
Application of Superconductors
- In research and development field
- In electrical machines, transformers, and cables
- In superconducting solenoids for low temperatures
- In electrical switching elements
Classification of Magnetism in Magnetic Materials
It has been observed that the motion of electrons around the nucleus, as well as about their own axes, creates magnetic moments in the atoms of a material. These magnetic moments produce magnetic field in an atom, which affect the nature of magnetism in the materials. This magnetism may be classified into the following three types:
- Diamagnetism
- Paramagnetism
- Ferromagnetism
- Diamagnetism: The term “diamagnetism” may be defined as a magnetism in which a material gets weakly magnetized in the direction opposite to that of the applied field. The diamagnetism is originated due to the motion of the electrons in circular and elliptical orbits around their nuclei. Each electron moving in an orbit is equivalent to an electron current flowing in a closed loop. This current produces a magnetic field in a direction at right angles to the plane of the orbit. According to the Law of electromagnetism, this magnetic field induces a magnetic moment in the atom in a direction opposite to it. We know that material contains a large number of electrons and the orbits of these electrons are randomly oriented in space. Therefore the magnetic moments of all such electrons are also randomly oriented.As a result of this, the magnetic moments of all the electrons get cancelled. Thus the net magnetism in the material is zero.
It has been observed that when an external field is applied to a material, its electrons experience a force which changes their angular momentum. It also changes the orientation of their magnetic moments in the atoms. As a result of this, some of the magnetic moments change their orientation with respect to the direction of the field, while others remain unchanged. The net effect of this phenomenon is that the magnetic moments in an atom do not cancel each other completely. But some magnetism is still left in the atom. This magnetism, which is in the opposite direction to that of the external field, is known as diamagnetism.
It will be interesting to know that diamagnetism is present in all the material since the motion of electrons is a universal phenomenon. But the materials, in which the diamagnetism is present to a large extent, are copper, gold, germanium, silicon etc.
- Paramagnetism: The term “paramagnetism” may be defined as a type of magnetism in which the material gets weakly magnetized in the same direction as that of the applied field. The materials, which exhibit paramagnetism are alkali metals, transition metals and rare earth elements (i.e. lanthanides and actinides).
The origin of paramagnetism arises due to the presence of permanent magnetic moments in the atoms or molecules. The permanent magnetic moments in single atom is created due to:
- Motion of an electron in circular and elliptical orbits around the nucleus (called orbital normal motion)
- Motion of an electron about its own axis (called Spin motion)
It has been observed that the magnetic moment created due to the orbital motion of electrons, disappear due to the effect of electric field of the neighbouring charges. But magnetic moments due to spin motion of electrons remain unaffected by this field. It will be interesting to know that the magnetic moments are composed of two groups. One of the groups contains electron with clockwise spin and the other electrons with anticlockwise spin. In the absence of external field, these magnetic moments are randomly distributed. Or in other words they have no mutual interaction among them.
As a matter of fact, when the external field is applied, the magnetic moments tend to line up in the direction of field. If there is no opposing force, all the magnetic moments line up, and materials acquires a large magnetization. But the thermal agitation of the atoms opposes such a tendency of the magnetic moments, and keeps them at random. This results in a partial alignment of the magnetic moments in the direction of the external field and the material is weakly magnetized.
- Ferromagnetism: The term “ferromagnetism” may be defined as a type of magnetism in which a material gets magnetized to a very large extent in the presence of external field. The direction in which the material gets magnetized is the same as that of the external field. The origin of ferromagnetism arises due to the presence of permanent magnetic moments in the atoms or molecules of the material. It has been observed that when the external field is applied, the magnetic moments line up in the same direction as that of the external field. This alignment takes place in the same way as in paramagnetism.
In paramagnetism, the magnetic moments are unable to align themselves in the direction of the field, due to thermal agitation between the atoms. But in ferromagnetism, there is no such force, which may oppose the alignment of magnetic moment. Thus the material is magnetized to a very large extent. Some of the materials, which possess ferromagnetism are iron, cobalt, nickel etc. The relative permeability () of these materials is very high i.e. to the order of 105.
Ferrimagnetism
We have already discussed that if all the magnetic moments line up in the direction of the field, the substance is known as ferromagnetic. We have also discussed that in ferromagnetic materials, the magnetic moments are composed of two groups, which consist of electrons with opposite spin directions. It has been observed that in some materials if both the groups are aligned in opposite directions and their magnetic moments are equal, the substance is known as antiferromagnetic. In these materials, the net magnetization is zero.
However, the magnetic moments of the two groups may not be equal if the substance consists of two different elements. As a result of this, complete cancellations of the magnetic moments do not take place and it results in some net magnetization. This phenomenon is known as ferrimagnetization. The materials, which exhibit this type of behaviour are known as ferromagnetic materials or simply ferrites.
Ferrites
These are complex compounds of various metals and oxygen which exhibit the phenomenon of ferromagnetism. They have a chemical formular of the form MgAl2O4. This indicates that the ferrite MgAl2O4 is a compound of magnesium and aluminium oxides. The other ferrites can be formed by replacing magnesium by other divalent metals (i.e. metals having two valence elements). The commonly used divalent metals are iron, copper, nickel, cobalt, zinc, cadmium by ferric ions, which is trivalent metal (i.e. metal having three valence electrons).
It will be interesting to know that the ferrite, so obtained, is non-magnetic, what when other metals, mentioned above, are used the ferrites obtained are magnetic and have a high permeability. The ferrites have a very high resistivity (greater than 105 ohm-cm) and extremely low dielectric loss. They have low eddy current loss, but large hysteresis loss. These days, the ferrites are widely used in high frequency transformers and inductors. They are also used in microwave applications and as computer memory core elements.
Piezoelectricity
The term “piezoelectricity” may be defined as the ability of a material to develop charge on its surface when it is mechanically stress. This effect is observed in those dielectric materials which have permanent dipoles in them. These dipoles get polarized, when the material is stressed and hence develops a charge on its surface. Similarly, when a dielectric material possessing permanent dipoles, is subjected to an electric field and produces a mechanical strain. This process is known as inverse piezoelectric effect. This effect is generally, observed in all ferroelectric materials.
Some of the important piezoelectric materials are Rochelle salt, barium titanate, quartz, lead, zinconate titanate etc. These materials are used in transducers, which are used to convert electrical energy into mechanical energy and vice versa. They are also employed in microphones phonograph pick ups, strain gauges etc.
